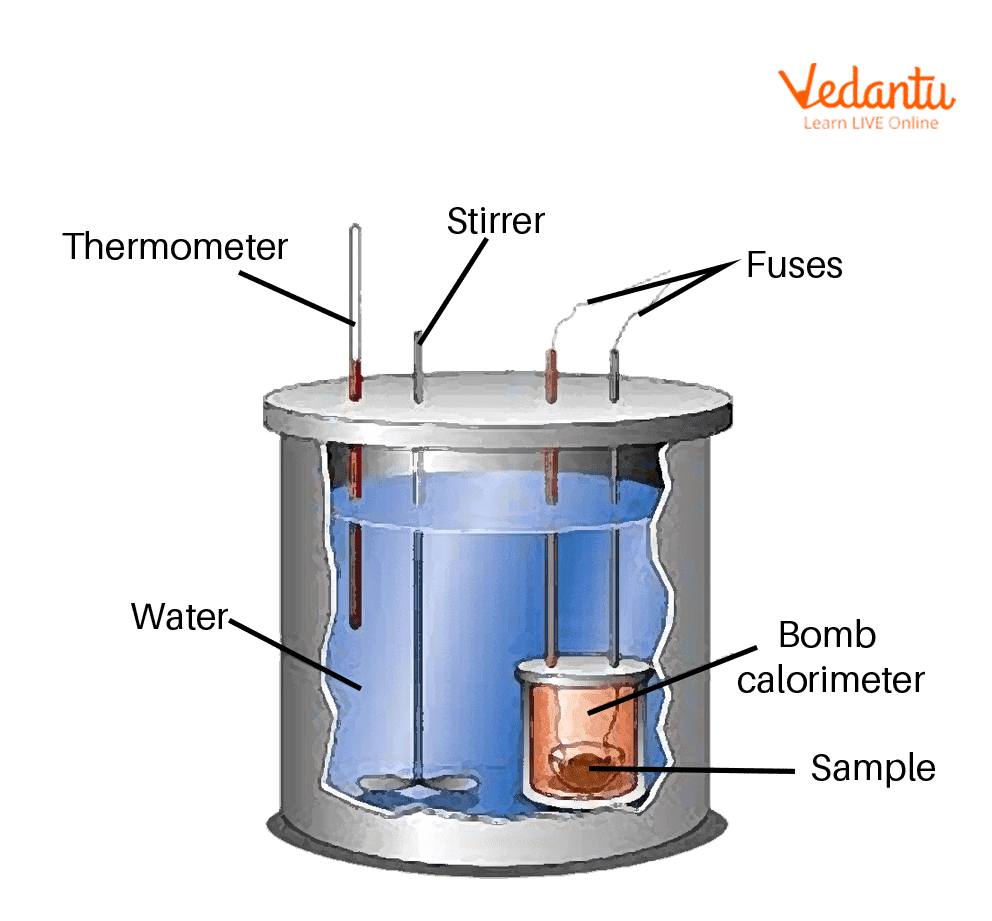
How Calorimeter Function . a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. It is used to measure the. a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of.
from www.vedantu.com
a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. It is used to measure the. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained.
Bomb Calorimeter Learn Important Terms and Concepts
How Calorimeter Function a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. It is used to measure the. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. a calorimeter is a device through which we can make the heat measurements necessary for calorimetry.
From chem.libretexts.org
6.3 Calorimetry Chemistry LibreTexts How Calorimeter Function It is used to measure the. a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in. How Calorimeter Function.
From www.thoughtco.com
Calorimeter Definition in Chemistry How Calorimeter Function calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. It is used to measure the. a calorimeter is a device through which we can make the heat measurements necessary for. How Calorimeter Function.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson How Calorimeter Function a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It is used to measure the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter is a device through which we can make the heat measurements. How Calorimeter Function.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness How Calorimeter Function a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. It is used to measure the. calculate heat, temperature change, and specific heat after thermal equilibrium is reached. How Calorimeter Function.
From ar.inspiredpencil.com
Calorimeter Diagram How Calorimeter Function It is used to measure the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. How Calorimeter Function.
From www.youtube.com
Calorimetry calculation YouTube How Calorimeter Function a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter. How Calorimeter Function.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Calorimeter Function calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. It is used to measure the. a calorimeter is a device through which we can make the heat. How Calorimeter Function.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses How Calorimeter Function a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. It is used to measure the. a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. a calorimeter is a device used to measure the amount of heat involved in. How Calorimeter Function.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube How Calorimeter Function calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy. How Calorimeter Function.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure How Calorimeter Function a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. It is used to measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is. How Calorimeter Function.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle How Calorimeter Function calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. It is used. How Calorimeter Function.
From www.youtube.com
Barrel Calorimeter (Function, Types, Features, Structure, Working How Calorimeter Function a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a. How Calorimeter Function.
From scienceinfo.com
Calorimeter Definition, Types and Uses How Calorimeter Function It is used to measure the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. calorimeter, device for measuring the heat developed during a mechanical, electrical,. How Calorimeter Function.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube How Calorimeter Function calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. . How Calorimeter Function.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co How Calorimeter Function calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. It is used to measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or. How Calorimeter Function.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types How Calorimeter Function a calorimeter is a device through which we can make the heat measurements necessary for calorimetry. It is used to measure the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. How Calorimeter Function.
From glossary.periodni.com
Download calorimeter.png image from How Calorimeter Function a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained.. How Calorimeter Function.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica How Calorimeter Function a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. It is used to measure the. a calorimeter is a device through which we can make the. How Calorimeter Function.